From Oil Sands to Open Fields: A Scientist-Rancher’s Defense of Agriculture and the Haber-Bosch Miracle
For those of you who do not know, I am an aspiring professional homesteader and rancher in south central Alberta. One of my passions in this latest chapter of my life is learning how to become as self sufficient as I possibly can in terms of my families’ food requirements.
Seven years ago, I embarked on this new journey, which was in part driven by external market and political forces, as well as inspired by my desire to achieve greater freedom in my life. Note that this career transition occurred after a mere 12 years out of graduate studies, as I had grown tired of corporate and national politics, while finding myself longing for a return to my blue collar rural roots.
A major factor in my growing discontent was the demoralization that both I and my peers felt, working in oil sands technology development.
Being bombarded on a daily basis by falsities on the nature of our business and its negative impacts on Canadians in general, while being told to not speak up or to defend ourselves for fear of retaliation, made me increasingly long for a vocation in which I could feel empowered and proud of my sweat equity.
A prime example of this capitulation to activist smear tactics was the invasion of Suncor’s crown jewel oil sands mining, extraction and upgrading facility just outside of Fort McMurray in the fall of 2009. Greenpeace activists sailed down the Athabasca River from Fort McMurray and landed along the shores of Suncor’s property bordering the river and proceeded to enter numerous extraction facilities and chain themselves to conveyors and ore crushers.
This event, forced the entire 8,500 person workforce to cease their daily activities and over 350,000 barrels per day of light sweet synthetic crude production came off line for over a day.
The loss of revenue was in the tens of millions.
Suncor refused to press corporate charges even though Greenpeace activists had broken the Law and I was told by my Management that if I wanted to defend my industry or company, I should create a fake social media profile.
I was blown away by this experience and was angered by not being able to talk about the $100 plus million tailings pond reclamation project that I was leading at Base Plant. Even though I knew intimate details about our immense efforts and corporate commitment to progressively return the land back to Nature, I was told to stay quiet.
Fast forward to 2025 - I feel increasingly compelled to begin advocating for farmers and ranchers - now that cows are increasingly becoming the new coal.
Watching increasing activism in the European Union, United States, Australia and Canada, against western agricultural industries, I wish to speak truth to power as I see it as both a rancher and as a scientist.
So hear we go.
History shows that urbanization has been made possible as agricultural productivities have steadily increased over the modern era and that the enabling commodities that have made this all possible are hydrocarbons. With ever increasing productivity, a single farmer or ranchers has seen multiple orders of magnitude increase in the number of non-agrarian citizens that they are able to support in ever increasingly specialized roles. While it took over 4 people to produce excess food to support even 1 person in a non-agricultural activity prior to the Industrial Revolution, today 1 farmer can support hundreds.
The many discoveries that have given us the ability and know-how to unlock the geological energy beneath our feet, has in turn given rise to the mechanization of farm labor and the scalable production of synthetic nitrogen fertilizers.
Unfortunately, urbanization has also created historic levels of easily exploited agricultural naivety.
With fewer people engaged in food production, the knowledge of how things are, is increasingly absent from the general metropolitan population.
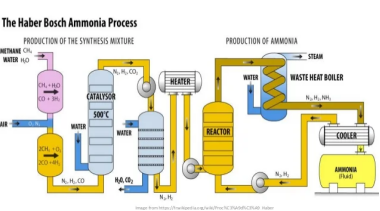
This article aims to remind us all of some of the facts of history specific to the Green Revolution and the introduction of synthetic nitrogen fertilizers made possible by the invention of the Haber-Bosch process. The Haber-Bosch process is in essence an air separation and natural gas steam reforming or coal gasification plant, which produces and combines purified streams of nitrogen gas (N2) and hydrogen (H2) extracted from hydrocarbons, to synthesize ammonia (NH3).
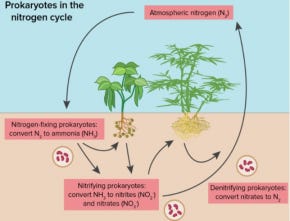
Figure 1. Bacterial mediated nitrogen cycle.
The Haber-Bosch process stands as one of humanity’s most transformative inventions, a triumph of science and engineering that reshaped agriculture, fueled global population growth, and redefined our relationship with the Earth’s natural systems. Breaking free from its dependence on natural rates of nitrogen fixation by soil bacteria or by atmospheric deposition, Homo Sapien achieved a historically unprecedented degree of autonomy from Mother Nature.
To fully appreciate its significance, we must first revisit the agricultural revolutions of the 17th and 18th centuries, which set the stage for the nitrogen crisis of the late 19th century.
From the guano trade to the ancient practice of summer fallow, humanity grappled with the challenge of maintaining soil fertility, a struggle that the Haber-Bosch process would ultimately resolve in a way that revolutionized food production and human progress.
The agricultural revolution of the 17th and 18th centuries marked a turning point in human history. Innovations such as crop rotation, improved plows, and selective breeding boosted yields and laid the foundation for modern farming.
Central to this transformation was the recognition that soil fertility, particularly nitrogen availability, was critical to sustaining agricultural productivity and to enhancing drought resistance in crops. Nitrogen, a key component of proteins and DNA, is essential for plant growth, but it is often scarce in forms that crops can readily use.
One of the earliest solutions to this problem was the guano trade, which emerged as a global phenomenon in the 19th century. Guano, the accumulated excrement of seabirds and bats (Figure 2), was rich in nitrogen and phosphorus, making it an exceptional natural fertilizer that is multiple times more potent per unit mass than cow or horse manure.
Figure 2. Guano deposit forming on an island off the coast of Peru.
By the mid-1800s, guano deposits, particularly from the Chincha Islands off the coast of Peru, became a cornerstone of industrial agriculture in Europe and North America. Farmers relied on guano to replenish depleted soils, boosting crop yields and supporting growing populations.
However, Peru’s White Gold was finite.
Figure 3. Mid-19th century Chincha island quano mining operation.
By the late 19th century, the best guano deposits were nearing exhaustion, and the logistical challenges of mining and transporting it across oceans made it increasingly costly and unsustainable.
The guano boom exposed a critical limitation: humanity’s dependence on naturally occurring nitrogen sources was a bottleneck that threatened to cap agricultural growth.
Parallel to and pre-dating the guano trade, farmers employed the ancient practice of summer fallow to restore soil nitrogen. In this method, fields were left unplanted for a season, allowing natural processes like atmospheric nitrogen oxide deposition and nitrogen fixation by soil bacteria to replenish nutrient levels.
While effective to an extent, summer fallow was inefficient, reducing the amount of arable land in production and limiting yields. For example, in semi-arid landscapes like Ukraine and the North American Prairies, anywhere from 20% to 50% of arable landscapes were rotated between summer fallow and active production.
Figure 4. Examples of croplands under summer fallow.
Summer fallow, also required one or more plowing or harrowing treatments per season to keep weeds from going to seed, had a negative consequence of accelerating the deep oxidation of humic materials (aka carbon content) present in the soil. In the long run, this practice diminished the moisture retention capacity of the soil and increased the risks of crop failure due to even minor droughts.
Prime examples of such crop failures in the 20th century included the North American Dust Bowl of the 1930s and the Ukrainian Holodomor (aka death by hunger) in 1931 - 32. Both disasters involved improper farming practices, made much worse by a multi-year drought.
As populations grew and urbanization accelerated in the late 19th century, the demand for food outstripped the capacity of these traditional methods. The world faced a looming nitrogen crisis: without a reliable and scaleable source of nitrogen, agriculture could not keep pace with human needs.
Enter Fritz Haber and Carl Bosch, two German scientists whose collaboration would change the course of history. In the early 20th century, the scientific community was racing to find a way to synthesize ammonia, a nitrogen-rich compound that could serve as a precursor to fertilizers and explosives.
Nitrogen gas (N₂), which makes up roughly 78% of Earth’s atmosphere, is abundant but chemically inert, requiring immense energy to break its strong triple bond and convert it into usable compounds. The Haber-Bosch process consumes 1–2% of global energy, equating to 6 – 12 EJ annually, with ~70–75% of this energy derived from hydrocarbons (primarily natural gas for steam methane reforming, SMR).
Earlier attempts to fix nitrogen artificially had been inefficient and impractical for large-scale production. Fritz Haber, a brilliant chemist, developed a breakthrough in 1909 by demonstrating that nitrogen and hydrogen gases could be combined under high pressure and temperature, in the presence of a magnetite (Fe₃O₄) - alumina (Al2O3) stabilized catalyst, to produce ammonia (NH₃).
His laboratory-scale process was a proof of concept, but it was Carl Bosch, an engineer at the chemical company BASF, who transformed Haber’s discovery into an industrial reality. Bosch overcame formidable technical challenges, including designing reactors capable of withstanding extreme conditions and developing cost-effective catalysts.
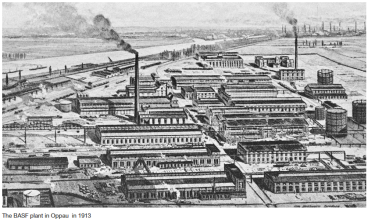
By 1913, the first commercial Haber-Bosch plant was operational, marking the dawn of industrial ammonia production.
Figure 5. Haber-Bosch process flow diagram.
The irony of the Haber-Bosch process lies in its original purpose. Developed in the context of early 20th-century geopolitics, the process was initially driven by the need for nitrogen-based explosives, such as ammonium nitrate, to fuel military ambitions.
Germany, in particular, sought to secure a domestic source of nitrates for munitions, reducing reliance on imported Chilean saltpeter (sodium nitrate). Yet, what began as a tool for warfare became a cornerstone of human progress. The ability to produce ammonia on an industrial scale revolutionized agriculture by providing a virtually limitless supply of synthetic nitrogen fertilizers, fundamentally altering humanity’s relationship with the natural world.
The Haber-Bosch process is nothing short of a modern miracle, because it allowed humanity to by-pass the limitations of the natural nitrogen cycle. In 2024, ammonia stands as the second most common manufactured chemical, with annual production estimated at 195 million tonnes.
In nature, nitrogen is fixed primarily by certain bacteria and lightning strikes, which convert atmospheric nitrogen into compounds like ammonia and nitrates that plants can absorb.
This natural process is slow and insufficient to meet the demands of intensive agriculture. Before Haber-Bosch, farmers were constrained by the availability of organic fertilizers like manure or guano and the inefficiencies of practices like summer fallow.
Figure 6. First Haber-Bosch plant in history - BASF plant in Germany 1913.
By synthesizing ammonia from atmospheric nitrogen, the Haber-Bosch process decoupled agriculture from these natural constraints. Synthetic fertilizers could now be produced in vast quantities and applied directly to fields, dramatically increasing crop yields. For example, the application of nitrogen fertilizers enabled some farmers to grow multiple crops per year on the same land, eliminating the need for summer fallow and maximizing arable land use.
Crops like wheat, corn, and rice, which are staples for billions, saw unprecedented productivity gains. Between 1900 and 2000, global food production increased by several orders of magnitude, largely due to the widespread adoption of Haber-Bosch-derived fertilizers. The impact extended beyond agriculture to adjacent ecosystems. Wetlands, forests, and grasslands benefited indirectly as agricultural expansion reduced pressure on marginal lands.
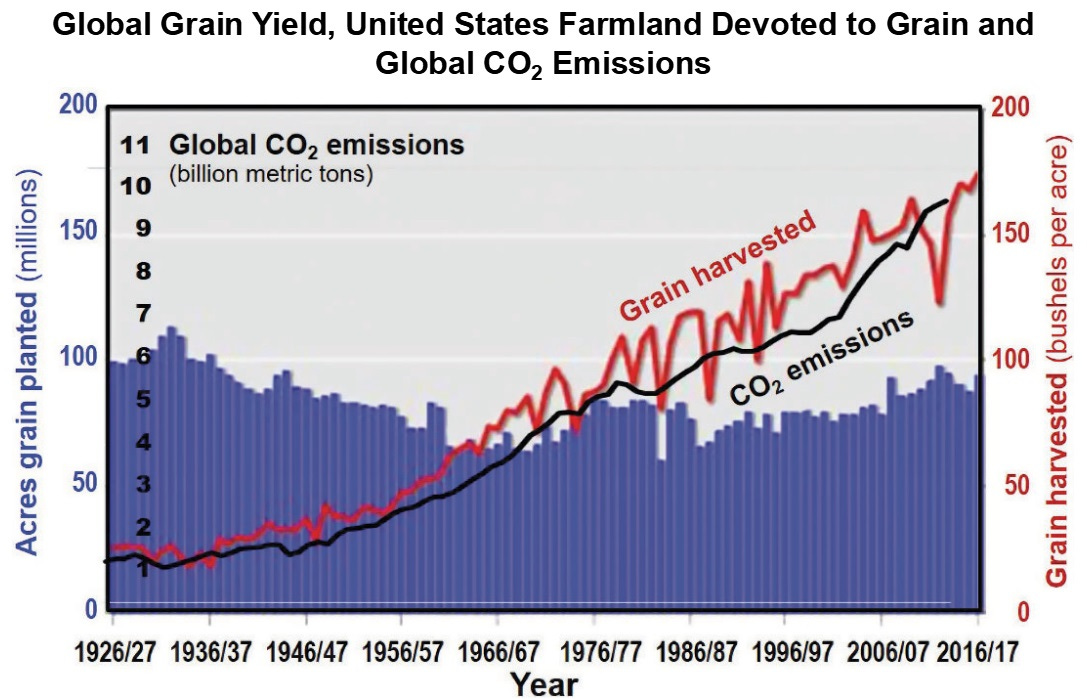
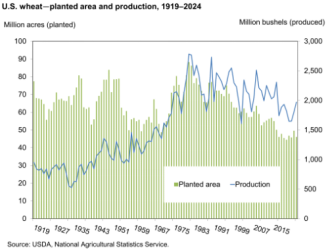
Figure 7. United States wheat production and cultivated land under wheat production.
The positive impact of the Haber-Bosch process became widespread especially in the post WWII era and Figure 7 shows this influence on wheat production in the United States. Note that there is more than a 200 % increase in production by 2020 relative to 1920, yet land allocated to wheat production decreased by 20 million acres.
By intensifying production on existing farmland, the Haber-Bosch process helped preserve natural habitats that might otherwise have been converted to agriculture to meet food demands.
This is not to say that all environmental outcomes were positive, but the process’s ability to concentrate productivity on smaller land areas was a significant achievement in the context of a growing global population, while preserving wild spaces.
While some narratives focus on the environmental and social challenges associated with the Haber-Bosch process, a common sense perspective emphasizes its unparalleled contribution to human progress. The process is often credited with enabling the global population to grow from 1.6 billion in 1900 to over 7 billion today.
Without synthetic fertilizers, it is estimated that nearly half the world’s population would face starvation, as natural nitrogen nutrient sources could not sustain modern agricultural yields.
The Haber-Bosch process has been a linchpin of the Green Revolution, which introduced high-yield crop varieties and modern farming techniques in the mid-20th century, further amplifying food production.
The process also democratized access to food.
By making fertilizers affordable and widely available, it empowered farmers in developing regions to increase yields and improve livelihoods. Countries like India and China, which faced chronic food shortages in the early 20th century, transformed into agricultural powerhouses, in large part due to their production of synthetic nitrogen.
This productivity boost not only reduced hunger but also fueled economic growth, as surplus food production freed up specialized labor supporting urbanization and industrialization. Moreover, the Haber-Bosch process exemplifies human ingenuity’s ability to solve existential challenges. At a time when the world faced a nitrogen crisis, science and engineering delivered a solution that transcended natural limits.
The collaboration between Haber’s theoretical brilliance and Bosch’s practical engineering underscores the power of interdisciplinary innovation. Their work laid the foundation for modern chemical engineering, influencing industries far beyond agriculture, from pharmaceuticals to materials science.
The Haber-Bosch process remains a cornerstone of modern civilization.
Today, Homo Sapien produce almost 200 million tons of ammonia annually, supporting roughly 50% of global food production. Its impact is so profound that it has been described as the most important invention of the 20th century, rivaling even electricity or antibiotics in its contribution to human welfare.
By liberating agriculture from the constraints of the nitrogen cycle, it has enabled humanity to thrive in ways previously unimaginable.
From the guano trade and summer fallow to the industrial synthesis of ammonia, humanity’s journey to overcome nitrogen scarcity reflects our capacity to adapt and innovate. Its legacy is one of progress, feeding billions, while transforming and preserving ecosystems. The Haber-Bosch process is not just a scientific achievement; it is a modern miracle that continues to shape the world for the better.
Please consider attending either in person or by livestream, my September 25th keynote address with the Friends of Science in Calgary Alberta:





Excellent article. I understand your frustration with the environmental activists. My former career was in the field of offshore seismic surveys. I remember in particular one summer in the mid-90’s where they attempted to disrupt our operations in Europe and in one notable instance, it showed their hypocrisy.
In this case, they used rigid inflatable boats to come within 200-300 meters of the survey vessel an attempt to steal equipment used for positioning. In the background were the freighter used to transport them and a helicopter to guide them to where they needed to go.
Best of luck in your new and future endeavours.
Excellent summary of a crucially important agricultural technology of which too few are aware. No mention was made of it in my 13 years at school. As a geologist coming from a farming background, I was fortunate to be one exposed to it.